In the pharmaceutical industry, Quality Assurance (QA) is essential for ensuring that products are manufactured and delivered to a safe and consistent standard, adhering to all guidelines, standards and stringent regulatory requirements set upon them. A challenge for all Pharmaceuticals is then how to effectively manage the whole Quality Assurance process as smoothly as possible whilst operating across multiple teams and departments; with the end goal of releasing as many batches on time and right first time.
This is an area where Olas’s application development department have offered our services to numerous Pharmaceuticals over the years, delivering bespoke Batch Tracker tools to log, manage and report on batches, from the moment they leave the processing line, to the moment the Qualified Person’s (QP) releases and certifies them.
Key goals of these projects have been to create a single tool that can be used across departments at all stages in the process. Providing easy to use, single versions of the truth, with full transparency of the overall process and visibility of at what stage any Batch currently stands in that process.
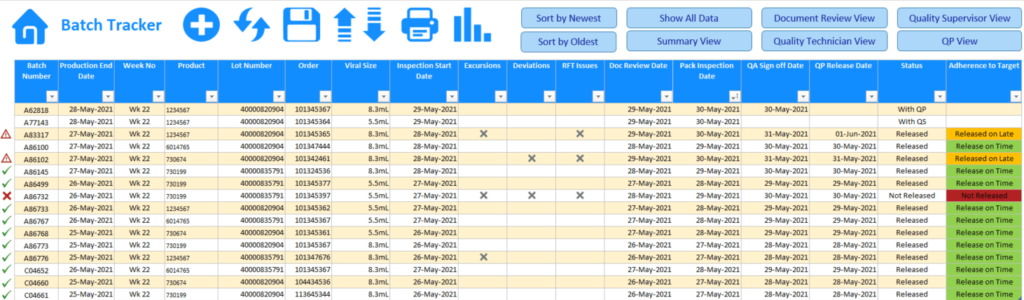
The Batch Tracker clock typically starts once production has completed and a Pack or Batch Number is first added to the log. From this point each team would be responsible for updating the log with key dates relating to the different stages that a batch must pass before successful QP release. Across all the different stages in the process, individuals would be required to record key dates and the status of those tasks relating to each batch item as they pass through the different steps right up until the final QP Release (Ex. Production > Laboratory > Packaging > Document Reviewer > Quality Assurance Technician > Quality Assurance Supervisor > QP Release).
Along this Batch Tracking journey information relating to Exceptions, Deviations, Right First Time (RFT) issues and Corrections can also be recorded against the batch, thus allowing for greater reporting of not just the adherence to target release times but of identifying more precisely the blockages that may occur along the way. This level of reporting can then in turn form the bases for putting Key Performance Indicators (KPI’s) in place for teams to strive for.
VARIATIONS OF TRACKERS
In a number cases, our trackers have been further extended to track shipments along the Supply Chain right up to the moment that a product is accepted by the client. Capturing key information relating to the transportation providers/methods, temperature exceptions, expiry dates, transportation times and final delivery dates.
- Batch Tracker
- Pack Tracker
- Shipping Operation Trackers
SAMPLES OF SOME OF THE KEY INFORMATION THAT MIGHT BE LOGGED
- Product Name
- Vial size
- Drug Concentration
- Material Number
- Pack Number
- Batch Number
- Lot Number
- Expiry Date
- Order Number
- Inspection lot
- PBR Date
- Production Start Date
- Production End Date
- Doc Review Date
- Pack Inspection Date
- QA Sign off Date
- QP Release Date
- Excursions
- Deviations
- RFT Issues
- Corrections
TECHNOLOGIES FOR CONSIDERATION
The Olas development team are highly experienced in delivering automated solutions across the Microsoft suite of products. For these types of projects, the following would be the main technologies that we would be looking to utilise.
- MS Access (Frontend screens and backend database)
- MS Excel (VBA coding to power the automation)
- MS Excel Power Query and Power Pivot elements
- MS SharePoint\MS Teams Lists (the backend database)
- MS SQL Server (the backend database)
- MS Power BI (reporting)
SAMPLE SETUP
In a multiuser environment an MS Access database application would be our preferred solution for combining data input with automation features and security controls. With increasing corporate migration from traditional fileserver hosting/networked folders, more often than not we have been using MS Access for the frontend screens development and then utilising MS SharePoint for hosting the backend database. MS Excel would then be the most common tool for data reporting but increasingly we are seeing Power BI been used for this purpose.
- MS Acccess Frontend Applciation Screens (GUI Interface)
- MS SharePoint Hosted Backend Data (SharePoint Lists)
- MS Excel Utilised for Detailed Pivot Reporting Analysis
KEY GOALS\FEATURES OF OUR BATCH TRACKER PROJECTS
- Delivering a quality, professional and complete solution
- Clear, concise and easy to use data input and reporting elements
- One version of the truth
- An open system with visibility at all stages of the tracking process
- Greater visibility of pipeline work
- Improve cross departmental communication and team efficiency’s in working towards shared goals
- Internal process auditing
- A high capacity for further data analysis and trend analysis
- Allow team more time for analysis and value-added activities within department
- Mandatory fields to avoid incomplete data entries
- Conditional formatting
- Data validation rules
- Pre-set dropdown selections
- Inbuilt formula
- Pivot table/chart reporting
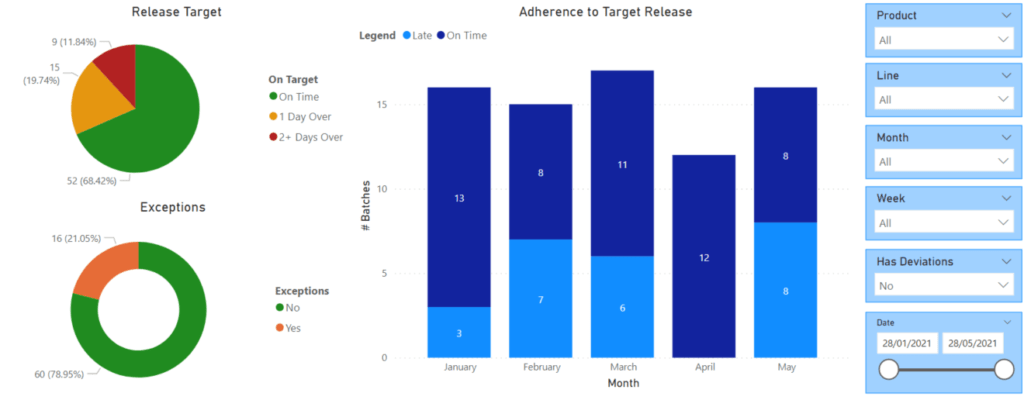
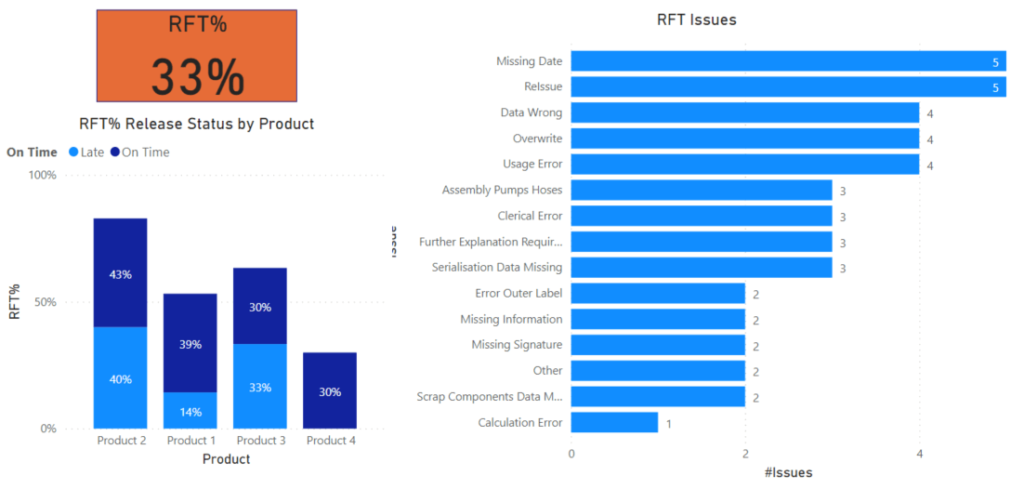
KEY AREAS FOR REPORTING
The following are examples of areas where reporting might be focused.
- Excursions (number and type)
- Deviations (number and type)
- Right First Time % and issues
- Right first time per stage
- QP Release times by product/line
- Certificate of Analysis
- Adherence to number of day release targets
- Incorporate organisation KPI’s
- On Time\Late
WOULD YOU LIKE TO ENQUIRE ABOUT HOW OLAS CAN ASSIST YOU IN DEVELOPING A BATCH TRACKING SOLUTION?
All our tools are built to our customer’s bespoke needs, leveraging our own experience in producing such solutions. Timings and costs would be dependent on the exact customer requirements and technologies being developed too.
To learn how our development can help design and develop a Batch Tracking solution tailored to your exact needs, just send an email to development@olas.ie and we will be back in touch promptly to arrange a call.
To find out more about our Application Development teams services, click HERE
Author: Fergal O’Connor, Senior Training Consultant, Application Development Department
CALL US FOR A FREE CONSULTATION WITH SOMEONE WHO SPEAKS YOUR LANGUAGE

